
Isotopes: These are atoms of the elements having the same atomic number but a different mass number. The atomic number is the number of proton or electrons in an atom of the element is called the atomic number. The mass number is the sum of a number of protons and neutrons in an atom of the elements is called the mass number. They differ in the chemical property but has the same physical property. These are atoms of the elements having the same mass number but different atomic numbers. The atomic number of argon 18 and an atomic number of calcium is 20. Calcium and argon have a mass number of 40. Calcium and argon are examples of a pair of Isobars. After the risks caused by the flammability of hydrogen became apparent, it was replaced with helium in blimps and balloons. Helium-oxygen breathing gas is often used by deep-sea divers at depths of seawater over 55 m (180 ft). Noble gases have several important applications in industries such as lighting, welding, and space exploration. Xenon is the only noble gas that may form compounds either with fluoride or oxide. They have a complete octet that makes them very stable, so they hardly react with other elements. Nobel gases conduct electricity and fluorescence which can be needed in many conditions to maintain a constant and safe environment. All noble gases are insoluble in water. Nobel gases are odourless, non-flammable, colourless, and monoatomic gas with low chemical reactivity. Radon is usually isolated from the radioactive decay of dissolved radium, thorium, or uranium compounds. Helium is sourced from natural gas fields that have high concentrations of helium in natural gas, using cryogenic gas separation techniques.  Neon, argon, krypton, and xenon are obtained from air in an air separation unit using the methods of liquefaction of gases and fractional distillation. Noble gases are also known as inert and rare gases. Their outer shell of valence electrons is considered to be "full", giving them little tendency to participate in chemical reactions, so they are considered the most stable elements of the periodic table. Radon is the only radioactive out of all and it's also not present in the Atmosphere.
Neon, argon, krypton, and xenon are obtained from air in an air separation unit using the methods of liquefaction of gases and fractional distillation. Noble gases are also known as inert and rare gases. Their outer shell of valence electrons is considered to be "full", giving them little tendency to participate in chemical reactions, so they are considered the most stable elements of the periodic table. Radon is the only radioactive out of all and it's also not present in the Atmosphere. Periodic table chemistry quiz series#
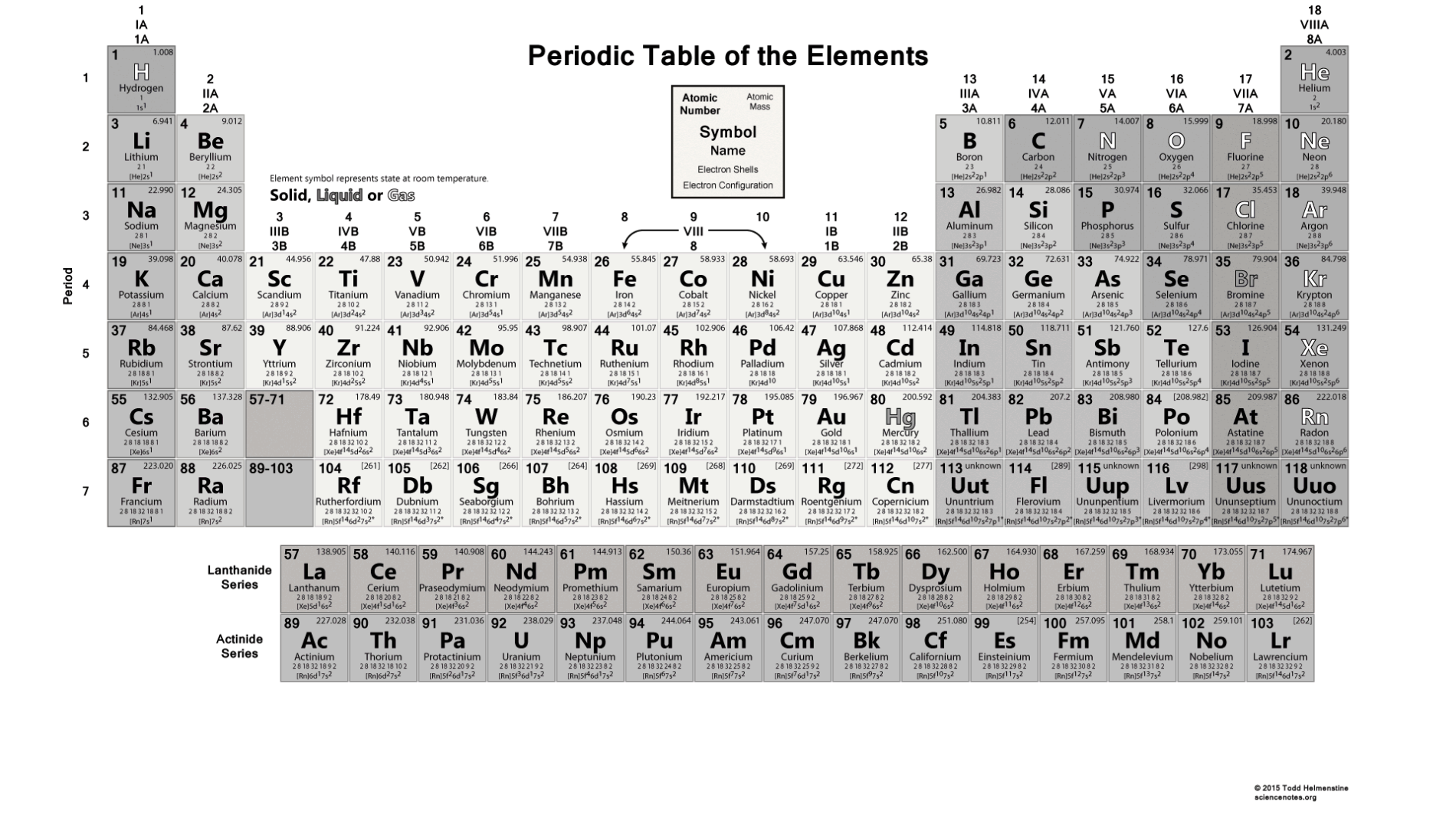
This group of a series of gases includes:.In the modern periodic table, group 18 belongs to noble gases.


Its properties are thus intermediate between those of chlorine and iodine.It is the third-lightest halogen and is a fuming red-brown liquid at room temperature that evaporates readily to form a similarly coloured gas.The electronic affinity of the Noble gases, Alkali metals, and Alkali earth metals are close to zero.Electron affinity decreases down the groups.Electron affinity increases from left to right in the Periodic Table.Electron affinity is the change in energy of a neutral atom (gaseous phase) when an electron is added to the atom to form a negative ion.Hence, argon has the lowest electron affinity.As a result, it doesn’t want to lose or gain any electrons.Argon has all filled orbitals as well as a filled valence shell.Make sure that you are familiar with periodic table of elements. Learn all informations about periodic table!Ĭhemical quiz, which will allow you to quickly memorize the symbols of chemical elements, its groups, periods, blocks and atomic numbers.








 0 kommentar(er)
0 kommentar(er)
